Positive Results for Phase 1 Data of mRNA Vaccine against Coronavirus
Good news for COVID- 19 patients!
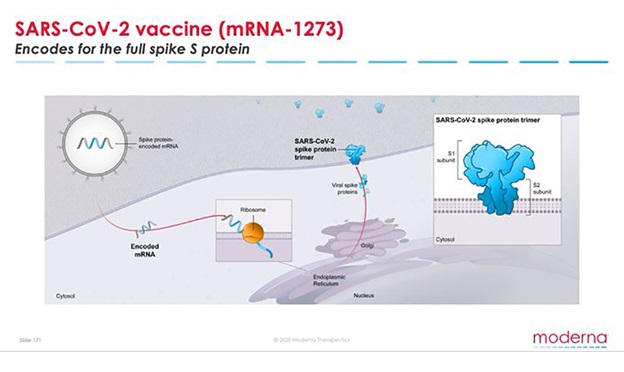
Moderna, a biotech company, working towards creating clinical therapeutics and vaccines, announced positive interim results for its phase 1 data of mRNA-1273, which is its revolutionary vaccine for curing coronavirus (Covid-19). The data, as of now, is accessible for 25ug and 100ug levels for ages 18 to 55. In light of the interval Phase 1 information, the Moderna-drove Phase 2 investigation will be corrected to examine two portion levels, 50 µg, and 100 µg, with the point of choosing a portion for vital examinations.
If the company combines the medicine that is responsible for preventing similar infection of the lungs at a portion that showed the same level of antibodies, then the data can easily materialize the fact that mRNA can be used to treat and prevent the novel coronavirus (Covid-19) and can help in advancing the chances of creating a successful vaccine for the virus. The team has been able to focus on starting the study for the next phase to file a BLA.
Moderna is a biotech firm that uses mRNA technology to cater to transformative vaccines for patients. The staff at Moderna has been successful in designing vaccines that are prophylactic and were used to treat many infectious diseases. Around 14000 candidates were enrolled for the company’s clinical studies for vaccines. The benefits of involving mRNA approach for these prophylactic vaccines is their ability to include variable mRNAs into a particular vaccine and then aid recovery to fight pandemic diseases, as well as to produce alertness to the platform. Moderna has developed a well-integrated manufacturing factory.
Moderna has been able to manifest in nine developments of vaccines currently. They include vaccines for infections in the respiratory system, vaccines for delivery transmitted infections from mother to baby, and vaccines for viral infections that are highly prevalent.
Source URL




 :
:  +91 – 9999064250 | 9811604444 | 9811604424
+91 – 9999064250 | 9811604444 | 9811604424