Gandhi Medicos: Exporter & Wholesaler For Pharmaceuticals
The leading exporters and wholesaler of speciality medicines.
For Quality and Cost Effective Solution.
Exporter & Wholesaler in Pharmaceutical
The leading exporters and wholesaler of speciality medicines.
For Quality and Cost Effective Solution.

Gandhi Medicos
SPECIALISING IN LIFE-SAVING MEDICINES.
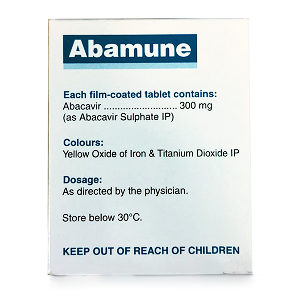
Gandhi Medicos -India, founded in 2006 in the capital of the India - New Delhi, is a one-point solution for all types of Cancer, Hepatitis-C, Hepatitis-B & HIV Medicines. We also deal in other medicines like Kidney & Liver Transplant, Injection & Life Saving Drugs, Nephrology Drugs, and other Pharmaceutical Capsules & Tablets from various companies. Gandhi Medicos also offers Health Monitors and Critical Care products. Gandhi Medicos sells products in Indian & international market.


THE MARKET LEADERS
At Gandhi Medicos, We deal with the Top Pharma Brands in the World.
Gandhi Medicos is the leading pharmaceutical Exporter, Supplier & Wholesale online Pharmacy of Generic Medicine.
Exporter, Supplier & Wholesale of Top Brands




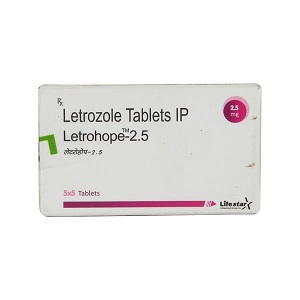
Top Inquired Products
MEDICINE RANGE
TOP CATEGORIES

LATEST PRODUCTS

0
Happy Customers
0
Products
0
Brands
0
Countries
READ HEALTHCARE NEWS
THE LATEST BLOGS BY GANDHI MEDICOS
Latest healthcare news and tips to stay fit .
CUSTOMERS REVIEWS
Featured Brands
Ordered medication from Gandhi Medicos and received it within hours! Talk about efficiency!
Thank you Gandhi medicos for helping out in dire circumstances. Had to ship hepatitis C meds to a friend overseas at the earliest. Didn’t want to send fake/overpriced meds. Got in touch with Mr Chetan at Gandhi Medicos and he made the whole process so seamless and trustworthy. They got meds delivered to their doorsteps in time. My friend in Europe sends her thanks to your team too!
Great experience at Gandhi Medicos! The staff is very professional and accommodating. They have a good selection of products, and the prices are competitive. Will definitely be coming back!"
You earned my loyalty dear Gandhi Medicos team! Your consistent quality and service over the years speak volumes about your trustworthiness.
Gandhi Medicos' commitment to prompt and secure delivery adds a layer of convenience, ensuring medications reach customers in a timely and efficient manner.
Trust in Gandhi Medicos is well-placed; their commitment to delivering genuine medicines has made them a reliable source for pharmaceutical needs.





 +91-9999064250 / 9811604424 / 9811604444
+91-9999064250 / 9811604424 / 9811604444
 8(800)100-47-90
8(800)100-47-90