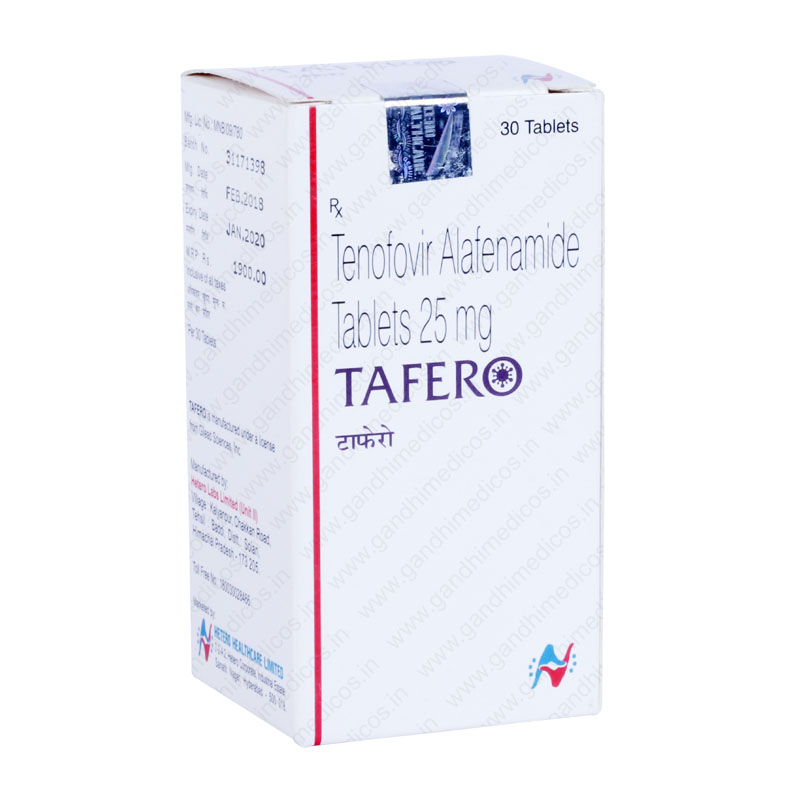
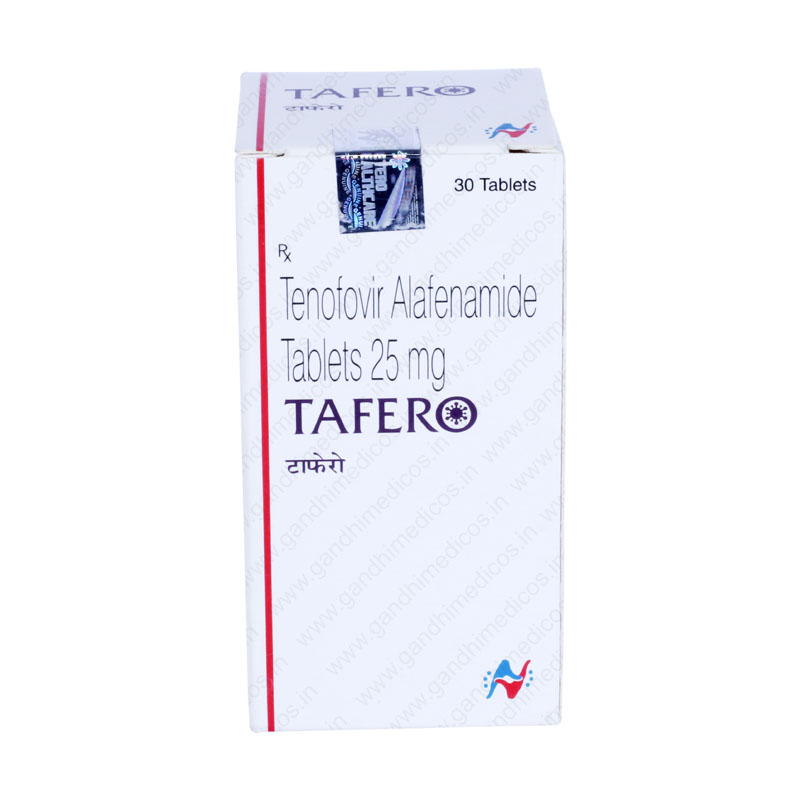
Tafero
| Product Name | Tafero 25 mg |
| Dose/Strength | 25 mg |
| Packaging Size | 30 Tablets |
| Brand | |
|---|---|
| Salt |
Thank you for your enquiry. We will get back to you shortly!

Lowest price guaranteed

Worldwide shipping

Genuine / original products

Fast Delivery
Category: Meds for HIV
Description
Tafero 25 mg
 Tafero 25 mg (Tenofovir Alafenamide) belongs to the class of anti-viral medications employed for managing human immunodeficiency virus (HIV) type 1 and Chronic Hepatitis B Viral (HBV) infections. Tafero 25 mg (Tenofovir Alafenamide) has many benefits including,
Tafero 25 mg (Tenofovir Alafenamide) belongs to the class of anti-viral medications employed for managing human immunodeficiency virus (HIV) type 1 and Chronic Hepatitis B Viral (HBV) infections. Tafero 25 mg (Tenofovir Alafenamide) has many benefits including,
- Prevention or slowing down of duplication process of the viruses
- Decreasing the proliferation of new viral cells in the system
- Clears the viral load from the system
- Increases the immunity power by enabling the growth of white blood cells (CD4) which protects against infection
- High efficiency with ten times lower dose than Tenofovir Disoproxil Fumarate
Administration of Tafero 25 mg (Tenofovir Alafenamide):
| Disease type | Underlying condition | Dosage regimen | Mode of administration |
| HBV | Compensated (with a functional system) liver disease | 1 tablet of 25 mg | Orally with food once daily |
| HIV-1 | Treatment-naïve | 1 tablet of 25 mg plus anti-retroviral drugs |
Tafero 25 mg (Tenofovir Alafenamide) dosage in different populations:
Mild, moderate, or severe kidney impairments: 1 tablet of 25 mg once daily
End-stage kidney disease: Not recommended
Mild liver impairments: 1 tablet of 25 mg once daily
Decompensated (without a functional system) liver disease: Not recommended
Side-effects of Tafero 25 mg (Tenofovir Alafenamide):
Common side-effects:
- Headache
- Tiredness (Fatigue)
- Nausea
- Pain in the stomach region and back
- Cough
Severe side-effects:
| Lactic acidosis (High lactic acid in the blood) | Liver complications |
| Unusual tiredness | Jaundice |
| Shortness of breath (Dyspnea) | Abdominal pain |
| Fast breathing | Urine in blackish-brown colour |
| Dizziness and Vomiting | Stools in a light colour |
| Cold or blue hands and feet | Nausea |
| Abnormal heartbeats | Loss of urge to eat |
Note: Please discuss with your doctor regarding the side-effects of Tafero 25 mg (Tenofovir Alafenamide) for more information.
Warnings associated with Tafero 25 mg (Tenofovir Alafenamide):
- Discontinuation of treatment leading to severe acute liver complications: Patients are strictly forbidden to stop their current dosing schedule as such decisions could cause severe problems in the liver.
- Risk of developing HIV-1 resistance in patients with HIV-1 coinfection: If patients already have HIV infection and have not received any cure, this drug might increase resistance to HIV medications.
- New onset or worsening condition of the kidney: If patients have any sort of problems in the kidney (end-stage renal disease), avoid using tenofovir-based medications for decreasing any chances of acute kidney failure.
- Missed dosage leading to resistance: Patients should take the dosage as prescribed by their doctor. If patients miss even a single dose, the viral cells may develop resistance against Tafero 25 mg (Tenofovir alafenamide).
- Drug interactions: Patients are obliged to inform about all medicines they are taking other than Tafero 25 mg (Tenofovir alafenamide).
- Pregnancy-related issues: Discuss the pregnancy and breastfeeding issues with your doctor.
Reviews (0)
Be the first to review “Tafero” Cancel reply
Related products
Abakast-L
Rated 5.00 out of 5


 Anti Cancer Drugs
Anti Cancer Drugs Hepatitis C
Hepatitis C Meds for HIV
Meds for HIV Ayurvedic Medicine
Ayurvedic Medicine Transplant Medicine
Transplant Medicine Respiratory System
Respiratory System +91-9999064250 / 9811604424 / 9811604444
+91-9999064250 / 9811604424 / 9811604444















Reviews
There are no reviews yet.